-

-
The fastest days for CE certification of respirators
1、 Why do masks need CE certificate for export to Europe For many products, CE certification is a necessary certificate to enter the European market. Most popular CE certified products are masks, many new ones In order to respond to the n...
-

-
How much is the cost of CE certification of respirator
1、 Why do masks need CE certificate for export to Europe For many products, CE certification is a necessary certificate to enter the European market. Most popular CE certified products are masks, many new ones In order to respond to the n...
-

-
How much does the mask cost to make EU CE certificate
Dust mask is mainly used to protect all kinds of particles. The body of the mask is usually made of filter material to prevent particles. It is fixed on the head by means of head strap, ear strap, etc. through forming a space separated from...
-

-
Why do masks need CE certification
Brief introduction of EU CE certification CE certification is the main requirement that constitutes the core of the European directive. In the resolution of the European Community on new methods of technical coordination and standards (85 /...
-
-
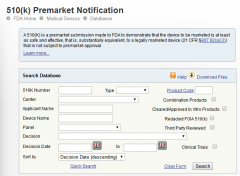
FDA certification inquiry
FDA certification query method: FDA query link: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRL/rl.cfm The following screen appears: 1. After entering the FDA registration and certification page, the left side is the enterprise...
-

-
Why do masks need FDA certification?
1、 Why do masks need FDA certification? N95 mask and medical mask belong to class II medical devices in the United States, and 510 (k) application is required. And other types of masks belong to class I medical devices, which can be regis...
-

-
FDA registration of medical masks
With the continuous expansion of new pneumonia in the United States, the U.S. government has had to make an urgent decision to exempt 27 U.S. enterprises from tariffs on more than 100 medical related products imported from China, such as ma...
-

-
How to register with FDA? How long will it take?
The mask is registered by FDA. The mask exported to the U.S. needs to be registered by FDA. The masks are divided into civil and medical categories. Different categories have different modes of selection. If the mask needs to be registered...
-

-
How to handle / process the CE certification of mask?
The mask is a kind of sanitary product, which is generally used to filter the air entering the mouth and nose by wearing it on the mouth and nose, so as to block harmful gases, smells and droplets from entering and leaving the mouth and nos...
-
![[respirator FDA certification] respirator FDA registration a [respirator FDA certification] respirator FDA registration a](/uploads/allimg/200326/1524351324-0-lp.png)
-
[respirator FDA certification] respirator FDA registration a
1、 Importance of mask FDA registration The mask is a kind of sanitary product, which is generally used to filter the air entering the mouth and nose by wearing it on the mouth and nose, so as to block harmful gases, smells and droplets fr...
推荐资讯
- 公示...
- 电池出口为何选择做CB认证...
- 干货 | 锂电池CB认证详解...
- 您对电池质量检验报告了...
- CCC&CQC傻傻分不清?快来...
- 你对印度BIS认证了解多少...
- 关于“CQC认证”App应用的...
- 【推荐】CQC标志认证最全...
- 注意出口日本!PSE认证安全...
- 阅读日本电器用品安全法...
- UN38.3检查意味着什么?必须...
- UN38.3锂电池检验报告是什...
- 带你进入美国ETL认证_ETL认...
- 介绍ETL认证...
- 印度BIS认证费BIS注意事项...
- 你对印度BIS认证了解多少...
- [有问题必答]3C认证17个常...
- 快看!最新的移动电源标准...
- 使用CB认证也有这个注意事...
- CB认证韩国电池KC认证接受...
众检检验Contact
业务咨询:400-1668-320
售后服务:0755-23720636
企业邮箱:info@cmczj-lab.com
深圳总部:广东省深圳市宝安区沙井街道大王山社区和二鸿奔工业区7栋
 收藏本站
收藏本站 联系众检
联系众检 网站地图
网站地图




